-
Новости
- ИССЛЕДОВАТЬ
-
Страницы
-
Группы
-
Статьи пользователей
-
Форумы
Rheumatoid Arthritis Diagnostic Test Market Size Projected to Reach USD 3,982.035 Million by 2032
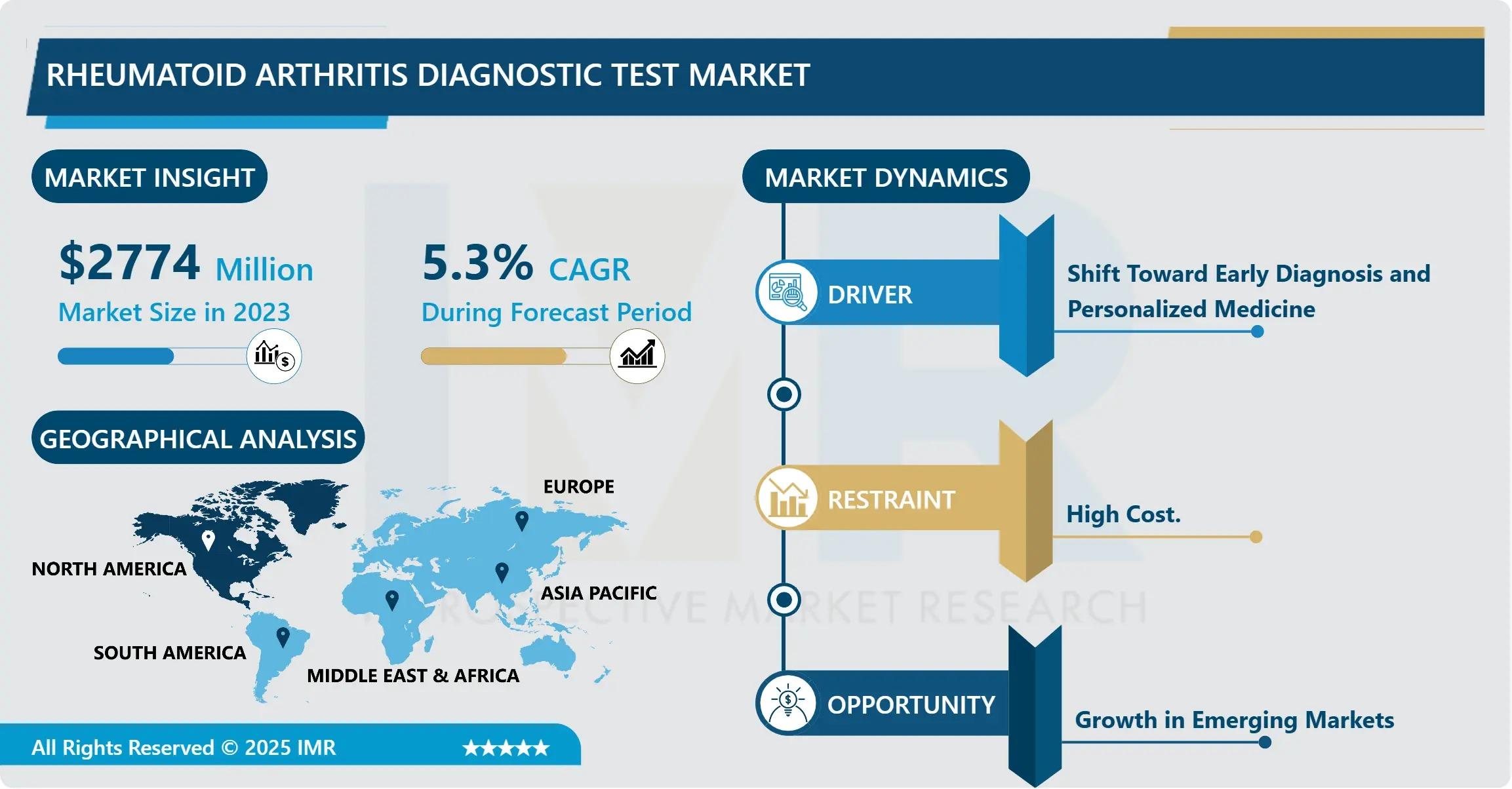
According to a new report published by Introspective Market Research, Rheumatoid Arthritis Diagnostic Test Market by Test Type (Serology Tests, Monitoring RA Treatment Efficiency Tests), Technology (ELISA, CLIA, Immunoturbidimetric Assay, Others), and End User (Hospitals, Diagnostic Laboratories, Ambulatory Surgical Centers), The Global Rheumatoid Arthritis Diagnostic Test Market Size Was Valued at USD 2,774.0 Million in 2023 and is Projected to Reach USD 3,982.035 Million by 2032, Growing at a CAGR of 5.3%.
Introduction / Market Overview:
The global rheumatoid arthritis (RA) diagnostic test market is a critical segment of the clinical diagnostics industry, focused on the early identification and management of a chronic autoimmune disorder that primarily affects the joints. Early diagnosis is paramount in RA, as it enables the initiation of disease-modifying therapies within the "window of opportunity"—typically the first three to six months—to prevent irreversible joint damage and disability. Modern diagnostic protocols have moved beyond traditional physical examinations to include a sophisticated array of serological markers, such as Anti-Cyclic Citrullinated Peptide (anti-CCP) and Rheumatoid Factor (RF), which offer high specificity and predictive value.
The market provides significant advantages over traditional symptom-based diagnosis by offering objective, quantifiable data that aids in differential diagnosis, distinguishing RA from other inflammatory conditions like lupus or gout. These tests are essential across the healthcare spectrum, from high-throughput hospital laboratories to specialized rheumatology clinics. As the medical community shifts toward precision medicine, the use of these diagnostic tools is expanding from simple detection to long-term monitoring of treatment efficacy, allowing for personalized dosage adjustments and improved patient quality of life.
Market Segmentation:
The Rheumatoid Arthritis Diagnostic Test Market is segmented into Test Type, Technology, and End User. By Test Type, the market is categorized into (Serology Tests [ESR, RF, anti-CCP, ANA, Others], and Monitoring RA Treatment Efficiency Tests). By Technology, the market is categorized into (Enzyme-Linked Immunosorbent Assay (ELISA), Chemiluminescent Immunoassay (CLIA), Immunoturbidimetric Assay, and Others). By End User, the market is categorized into (Hospitals, Diagnostic Laboratories, and Ambulatory Surgical Centers).
Growth Driver:
The primary driver for the Rheumatoid Arthritis Diagnostic Test Market is the increasing global prevalence of autoimmune disorders coupled with a rapidly aging population. Aging is a significant risk factor for RA, and as the global geriatric demographic expands, the volume of patients presenting with inflammatory joint symptoms is rising. Furthermore, increased awareness of early intervention among both clinicians and patients has led to a surge in routine screening. Healthcare systems are increasingly adopting "treat-to-target" strategies that rely heavily on regular diagnostic testing to achieve clinical remission, thereby driving consistent demand for both initial screening and follow-up monitoring assays.
Market Opportunity:
A significant market opportunity lies in the development of multiplex autoantibody panels and Point-of-Care (POC) testing solutions. While traditional ELISA-based lab tests are effective, there is an urgent demand for faster, decentralized testing that can be performed during a patient's initial clinic visit. Multiplex assays that simultaneously detect multiple biomarkers, such as anti-CCP and RF, provide a more comprehensive diagnostic profile in a single run, reducing the diagnostic delay. Companies that can successfully integrate these high-sensitivity assays into portable, user-friendly POC devices are well-positioned to capture the growing outpatient and remote-care segments.
Detailed Segmentation:
Title: Rheumatoid Arthritis Diagnostic Test Market, Segmentation The Rheumatoid Arthritis Diagnostic Test Market is segmented on the basis of Test Type, Technology, and End User.
Test Type The Test Type segment is further classified into Serology Tests and Monitoring RA Treatment Efficiency Tests. Among these, the Serology Tests sub-segment accounted for the highest market share in 2023. This dominance is attributed to the fact that serology—particularly anti-CCP and RF testing—is the clinical gold standard for the initial diagnosis of RA. These tests are integrated into global ACR/EULAR classification criteria, making them a mandatory part of the diagnostic workflow. The high sensitivity of modern assays allows for the detection of RA even in pre-symptomatic stages, ensuring a high volume of testing across both public and private diagnostic networks.
End User The End User segment is further classified into Hospitals, Diagnostic Laboratories, and Ambulatory Surgical Centers. Among these, the Diagnostic Laboratories sub-segment accounted for the highest market share in 2023. Most RA diagnostic testing requires high-throughput automated platforms and specialized expertise to interpret complex immunological results. Large-scale diagnostic laboratories benefit from economies of scale and often serve as referral hubs for clinics and smaller hospitals. Their ability to offer comprehensive "arthritis panels" that combine several serological and inflammatory markers makes them the preferred choice for primary care physicians and rheumatologists.
Some of The Leading/Active Market Players Are-
- Abbott Laboratories (USA)
- Siemens Healthineers AG (Germany)
- Thermo Fisher Scientific Inc. (USA)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Danaher Corporation (Beckman Coulter) (USA)
- Bio-Rad Laboratories, Inc. (USA)
- Euro Diagnostica AB (Sweden)
- Exagen Inc. (USA)
- Augurex Life Sciences Corp. (Canada)
- Svar Life Science AB (Sweden)
- Qiagen N.V. (Netherlands)
- Boditech Med Inc. (South Korea)
- Tulip Diagnostics (P) Ltd. (India)
- Getein Biotech, Inc. (China)
- Labcorp (USA)
and other active players.
Key Industry Developments
News 1: In September 2024, Exagen Inc. announced the expanded validation of its AVISECURE technology, which focuses on the detection of anticarbamylated protein (anti-CarP) antibodies. These antibodies are found to be elevated in RA patients, even those who are seronegative for traditional markers. This development marks a major step forward in closing the diagnostic gap for patients with "seronegative" RA, providing clinicians with a more robust toolset to identify the disease earlier and initiate aggressive treatment before joint erosion occurs.
News 2: In March 2025, Siemens Healthineers received regulatory clearance for an advanced automated high-throughput anti-CCP assay for its Atellica Solution platform. This new assay is designed to reduce turnaround times while maintaining high specificity for early RA. By integrating this test into high-volume laboratory workflows, Siemens is addressing the global shortage of specialized lab personnel and the increasing demand for rapid, accurate results in busy clinical settings, thereby improving the overall efficiency of RA care pathways.
Key Findings of the Study
- Dominant Segments: Serology tests remain the core revenue driver, with anti-CCP testing emerging as the most specific biomarker.
- Leading Regions: North America dominates the market share (~40%), while the Asia-Pacific region is the fastest-growing due to improving healthcare infrastructure.
- Key Growth Drivers: Rising geriatric population and the clinical shift toward early, aggressive "Treat-to-Target" protocols.
- Market Trends: Integration of AI for synovial imaging analysis and the rise of at-home/point-of-care screening kits.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Игры
- Gardening
- Health
- Главная
- Literature
- Music
- Networking
- Другое
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness